The concept of atomic theory dates back to ancient Greece, with Leucippus and Democritus often credited as early proponents. They proposed that everything is composed of small, indivisible particles called “atoms.”
Atomic theory is a foundational concept in science that posits that matter is composed of discrete units known as atoms. This theory has evolved significantly over centuries, from early philosophical ideas to a well-established scientific framework. The journey of atomic theory reflects our growing understanding of the fundamental nature of matter and the universe itself.
Understanding the origins of atomic theory is crucial because it highlights how human thought has progressed from abstract concepts to empirical science. By examining the historical development of atomic theory, we gain insight into the evolution of scientific ideas and the critical figures who shaped our modern understanding of the atomic structure.
Ancient Greek Philosophers
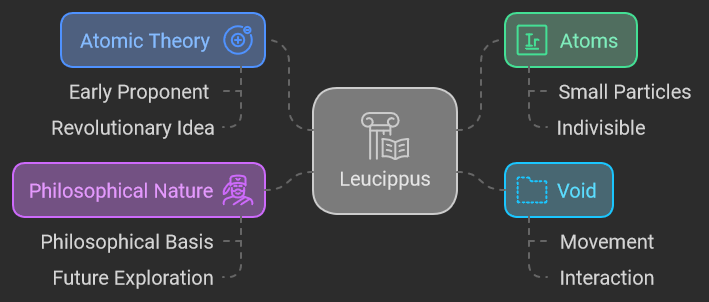
Leucippus (c. 5th century BCE)
Leucippus is often regarded as one of the earliest proponents of atomic theory. Operating in the realm of ancient Greek philosophy, Leucippus introduced the revolutionary idea that everything in the universe is composed of small, indivisible particles called “atoms.” According to Leucippus, these atoms move through the void, an empty space that allows them to interact and combine. His ideas were largely philosophical, lacking empirical evidence but setting the stage for future scientific exploration.
Leucippus’s concept of atoms and the void was groundbreaking for its time, laying the groundwork for later philosophical and scientific developments. His theory challenged the prevailing ideas of continuous matter and contributed to the early framework of atomic thought.
Democritus (c. 460 – c. 370 BCE)
Democritus, a student of Leucippus, further developed the atomic theory by expanding on his mentor’s ideas. He proposed that atoms are not only indivisible but also vary in shape and size, which influences the properties of different materials. Democritus’s theory suggested that these atoms are in constant motion and combine in various ways to form all substances in the universe.
Democritus’s contributions to atomic theory were more detailed than those of Leucippus, including the idea that the arrangement and movement of atoms determine the nature of matter. His work laid a foundation for future scientific exploration and had a lasting influence on subsequent developments in chemistry and physics.
Modern Atomic Theory
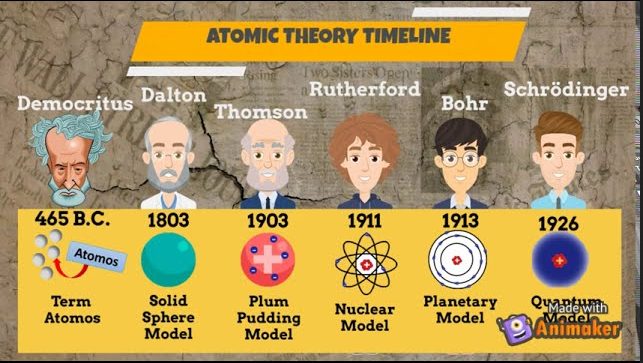
John Dalton (1766–1844)
John Dalton was a pivotal figure in the development of atomic theory during the early 19th century. Living through a period of significant scientific advancement, Dalton’s work marked a major leap from philosophical speculation to empirical science. His modern atomic theory provided a clearer, evidence-based understanding of the nature of matter.
Key Contributions:
- Atoms as the Basic Unit of Matter: Dalton proposed that all matter is composed of tiny, indivisible particles called atoms. This concept established atoms as the fundamental building blocks of matter, which was a significant departure from previous notions of matter being continuous.
- Unique Atoms for Each Element: Dalton’s theory included the idea that each element is made up of atoms of a specific type, unique to that element. For example, he posited that hydrogen atoms are different from oxygen atoms, which explained why elements combine in fixed ratios to form compounds.
- Chemical Reactions and Atom Rearrangement: Dalton introduced the idea that chemical reactions involve the rearrangement of atoms rather than their creation or destruction. This concept helped explain how substances change during chemical reactions, laying the groundwork for modern chemistry.
Dalton’s atomic theory had a profound impact on the field of chemistry, providing a systematic framework for understanding chemical reactions and the composition of matter. His work refined and expanded upon earlier ideas, setting the stage for future discoveries in atomic and molecular science.
Conclusion
The journey of atomic theory from its philosophical beginnings with Leucippus and Democritus to its empirical foundation laid by John Dalton illustrates a remarkable evolution in scientific thought. Leucippus’s initial ideas about indivisible particles and Democritus’s expansion on these concepts provided a philosophical basis for understanding matter. Dalton’s contributions transformed these ideas into a scientific framework, profoundly influencing the field of chemistry.
These developments in atomic theory are not just historical milestones but also foundational principles that continue to shape our understanding of the natural world. The evolution of atomic theory underscores the importance of both philosophical inquiry and empirical research in the advancement of science.
Resources for Further Reading
- “The Atom and the Universe” – Encyclopaedia Britannica
Link - “John Dalton’s Atomic Theory” – Chemistry LibreTexts
Link - “Democritus and the Atom” – Stanford Encyclopedia of Philosophy
Link - “A History of Chemistry” – Science History Institute
Link
FAQ :
1. What is atomic theory?
Atomic theory is the scientific concept that matter is composed of discrete units known as atoms. It explains that atoms are the fundamental building blocks of matter and that they combine in specific ways to form all substances. The theory has evolved from ancient philosophical ideas to a well-established scientific framework.
2. Who were the earliest proponents of atomic theory?
The earliest proponents of atomic theory were the ancient Greek philosophers Leucippus and Democritus. Leucippus introduced the idea that matter is made up of indivisible particles called atoms. Democritus expanded on this idea by suggesting that atoms have different shapes and sizes and are in constant motion.
3. How did John Dalton contribute to atomic theory?
John Dalton, in the early 19th century, significantly advanced atomic theory by providing a systematic and empirical framework. His key contributions included:
- Defining atoms as the basic units of matter.
- Proposing that each element consists of unique types of atoms.
- Introducing the idea that chemical reactions involve the rearrangement of atoms.
4. What is the significance of Dalton’s atomic theory in modern chemistry?
Dalton’s atomic theory laid the foundation for modern chemistry by introducing the concept of atoms as indivisible particles that combine in fixed ratios to form compounds. His ideas provided a systematic approach to understanding chemical reactions and the composition of matter, which has been crucial for the development of chemical science.
5. How did Democritus’s ideas differ from those of Leucippus?
While both philosophers contributed to early atomic theory, Democritus expanded on Leucippus’s ideas by introducing the notion that atoms have different shapes and sizes, affecting the properties of matter. Democritus also proposed that atoms are in constant motion and combine in various ways, adding more detail to the initial concept.
6. Why is understanding the history of atomic theory important?
Understanding the history of atomic theory is important because it highlights how scientific ideas evolve over time. It demonstrates the progression from philosophical concepts to empirical science and shows how early thinkers laid the groundwork for modern scientific discoveries.
7. Where can I find more information about the development of atomic theory?
For further reading, you can refer to the following resources:
- “The Atom and the Universe” – Encyclopaedia Britannica
- “John Dalton’s Atomic Theory” – Chemistry LibreTexts
- “Democritus and the Atom” – Stanford Encyclopedia of Philosophy
- “A History of Chemistry” – Science History Institute