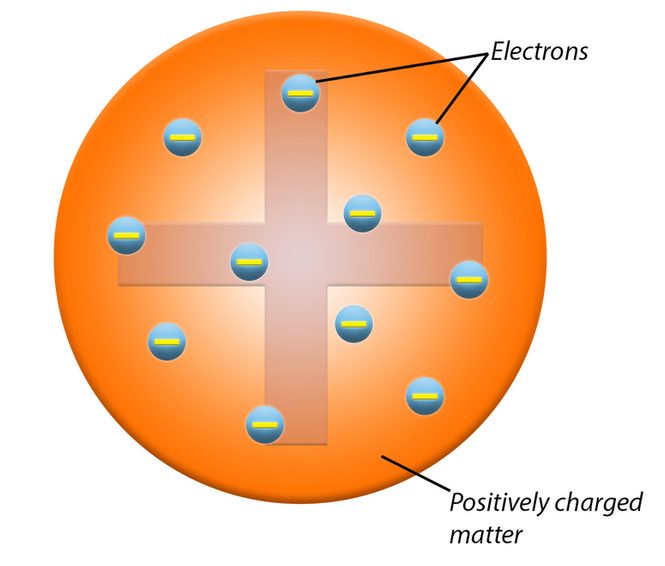
The plum pudding model was proposed by physicist J.J. Thomson in 1904, following his discovery of the electron. This model was an early attempt to describe the internal structure of the atom, offering a new perspective on atomic theory before the discovery of the nucleus.
The quest to understand the fundamental nature of matter has driven scientific inquiry for centuries. At the heart of this pursuit lies the concept of atomic models—frameworks that offer insights into the structure and behavior of atoms, the building blocks of all matter. These models have evolved significantly over time, each contributing to our expanding knowledge of the microscopic world.
One pivotal moment in this evolution came in the early 20th century with the discovery of the electron. J.J. Thomson, a British physicist, was instrumental in this breakthrough. In 1897, Thomson identified the electron, a negatively charged subatomic particle, through his experiments with cathode rays. This discovery challenged the existing views of atomic structure and highlighted the need for a new model to incorporate these newly discovered particles.
Overview of the Plum Pudding Model
Introduction to J.J. Thomson
J.J. Thomson (1856–1940) was a prominent figure in the field of physics whose work profoundly impacted our understanding of atomic structure. Renowned for his discovery of the electron, Thomson received the Nobel Prize in Physics in 1906 for his investigations into the conduction of electricity in gases. His work laid the groundwork for future atomic theories and set the stage for the development of the plum pudding model.
Development of the Model
In 1904, building on his discovery of the electron, Thomson proposed the plum pudding model as a way to conceptualize the internal structure of the atom. Prior to this, the atom was thought to be an indivisible entity. Thomson’s model represented a significant shift from this view. The plum pudding model suggested that atoms were composed of a diffuse sphere of positive charge with negatively charged electrons embedded within it. This model was designed to address the new understanding that atoms contained subatomic particles, providing a framework to explain their overall neutrality and structure.
Structure of the Plum Pudding Model
Positive Charge Distribution
In J.J. Thomson’s plum pudding model, the atom was envisioned as a sphere with a uniform distribution of positive charge. Thomson proposed that this positive charge formed a diffuse, amorphous sphere, much like a pudding. To illustrate this concept, he used the analogy of a plum pudding—a traditional dessert. Just as a plum pudding consists of a soft, spongy base with plums scattered throughout, Thomson’s model suggested that the atom had a “pudding” of positive charge with negatively charged electrons embedded within it. This analogy helped visualize how the positive charge was spread throughout the atom, counteracting the negative charge of the electrons.
Electrons Embedded
According to the plum pudding model, electrons were embedded within this positively charged sphere, similar to how plums are scattered throughout a pudding. These electrons were thought to be randomly distributed throughout the positive sphere. This arrangement was proposed to ensure that the atom as a whole remained electrically neutral. The negative charges of the electrons were balanced by the positive charge of the sphere, resulting in an atom with no overall electric charge. This distribution of electrons within the positive “pudding” was Thomson’s way of accounting for the atom’s neutrality and its internal structure.
Rationale Behind the Model
Neutral Atom Concept
The plum pudding model was designed to address a key question in atomic theory: how can atoms be electrically neutral if they contain negatively charged electrons? Thomson’s model proposed that the atom’s positive charge was spread evenly throughout the entire volume of the atom, much like a pudding with dispersed plums. By having the positive charge distributed in this manner, the negative charges of the electrons were counterbalanced, resulting in an electrically neutral atom. This concept was crucial for explaining why atoms did not exhibit a net electrical charge despite containing charged particles.
Supporting Evidence
Thomson’s experiments with cathode rays provided the experimental foundation for the plum pudding model. In his cathode ray experiments, Thomson discovered that cathode rays were composed of particles with a negative charge and a small mass, which he identified as electrons. His measurement of the charge-to-mass ratio of these particles supported the idea that electrons were components of the atom. Additionally, Thomson’s experiments demonstrated that these particles were uniformly dispersed throughout the atom, leading him to propose that the atom was a positively charged sphere with electrons embedded within it. This experimental evidence was instrumental in the formulation of the plum pudding model and provided a new perspective on atomic structure.
Limitations and Successors
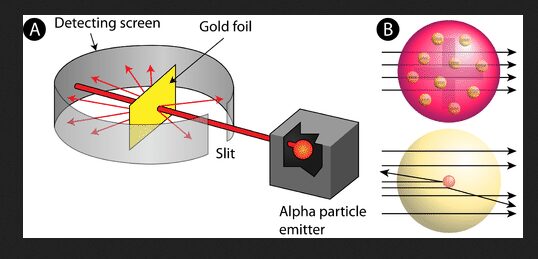
Rutherford’s Gold Foil Experiment
In 1909, Ernest Rutherford conducted a groundbreaking experiment using a thin foil of gold and a source of alpha particles. The setup involved directing alpha particles, which are positively charged, at the gold foil and observing their scattering patterns using a fluorescent screen and a detector. According to the plum pudding model, the alpha particles should have passed through the gold foil with minimal deflection because the positive charge was thought to be spread out diffusely within the atom. However, Rutherford’s results showed unexpected results: while most alpha particles passed through the foil with little deflection, a small fraction were deflected at large angles, and some even bounced back. These results suggested that the positive charge was not spread evenly but concentrated in a very small region within the atom, which contradicted the plum pudding model’s predictions.
Transition to the Rutherford Model
The surprising results from Rutherford’s gold foil experiment led him to propose a new model of the atom in 1911. This model, known as the Rutherford or nuclear model, posited that the atom consists of a dense, positively charged nucleus at its center, where most of the atom’s mass is concentrated. Electrons orbit this nucleus at a distance, similar to planets orbiting the sun. Rutherford’s model addressed the discrepancies observed in his experiment by placing the positive charge in a central nucleus, thus providing a more accurate representation of atomic structure and resolving the limitations of the plum pudding model.
Historical Significance
Impact on Atomic Theory
The plum pudding model was a pivotal step in the evolution of atomic theory. By proposing a structure for the atom that included subatomic particles, Thomson’s model marked a significant advancement from earlier, indivisible atom concepts. It provided a framework that inspired further research into atomic structure and helped scientists understand that atoms were not just simple spheres but complex systems containing multiple components.
The limitations of the plum pudding model, revealed by Rutherford’s experiment, spurred the development of more refined atomic models. The transition from Thomson’s model to Rutherford’s nuclear model paved the way for subsequent models, including Niels Bohr’s model, which introduced quantized electron orbits, and the modern quantum mechanical model. Each new model built upon the foundations laid by earlier theories, demonstrating the iterative nature of scientific understanding and the importance of experimental evidence in shaping our view of the microscopic world.
Conclusion
Summary of Key Points
The plum pudding model, proposed by J.J. Thomson in 1904, was a pioneering attempt to describe the structure of the atom. Thomson’s model depicted the atom as a sphere of positive charge with electrons embedded within it, akin to plums scattered throughout a pudding. This representation was an early effort to incorporate the newly discovered electron into atomic theory and to explain the atom’s overall neutrality. Despite its innovative approach, the plum pudding model was ultimately challenged by Rutherford’s gold foil experiment, which revealed a more complex atomic structure involving a concentrated nucleus. This shift in understanding marked a significant advancement in atomic theory.
Reflection on Scientific Progress
The development and eventual replacement of the plum pudding model illustrate the dynamic nature of scientific progress. Early models like Thomson’s were crucial in laying the groundwork for subsequent theories. While the plum pudding model was eventually superseded by the Rutherford model and later refinements, its introduction marked a key step in the journey towards a more accurate representation of atomic structure. These early models provided valuable insights and set the stage for future discoveries, demonstrating how scientific theories evolve over time through experimentation and refinement.
Resources for Further Reading
- HyperPhysics: Plum Pudding Model
HyperPhysics: Plum Pudding Model - Britannica: J.J. Thomson
Britannica: J.J. Thomson - Rutherford Gold Foil Experiment
Rutherford Gold Foil Experiment - History of Atomic Models
History of Atomic Models
FAQs
1. What is the plum pudding model? The plum pudding model is an early 20th-century atomic model proposed by physicist J.J. Thomson. It describes the atom as a sphere of positive charge with negatively charged electrons embedded within it, similar to plums in a pudding.
2. Who proposed the plum pudding model? The plum pudding model was proposed by J.J. Thomson in 1904, following his discovery of the electron in 1897.
3. What was the main idea behind the plum pudding model? The main idea of the plum pudding model was to explain the structure of the atom by distributing positive charge evenly throughout a spherical atom and embedding electrons within this positive sphere. This arrangement was intended to account for the atom’s overall neutrality.
4. Why was the plum pudding model eventually replaced? The plum pudding model was replaced after Ernest Rutherford’s gold foil experiment in 1909. Rutherford’s experiment showed that atoms have a dense, positively charged nucleus at their center, which contradicted the plum pudding model’s diffuse positive charge distribution. This led to the development of the Rutherford model of the atom.
5. What did Rutherford’s gold foil experiment reveal? Rutherford’s gold foil experiment revealed that most of the atom’s mass and positive charge is concentrated in a small, dense nucleus at the center of the atom. This finding contradicted the plum pudding model and led to the development of the nuclear model of the atom.
6. How did the plum pudding model contribute to atomic theory? The plum pudding model was significant in advancing atomic theory by incorporating the concept of subatomic particles into the atomic structure. It was an important step in the evolution of atomic models, providing a foundation for later theories, including Rutherford’s nuclear model and Bohr’s quantized orbits.
7. What was J.J. Thomson’s contribution to physics? J.J. Thomson is best known for his discovery of the electron in 1897. His work on cathode rays led to the identification of electrons as subatomic particles and significantly impacted the development of atomic theory. He also proposed the plum pudding model of the atom.
8. What are some limitations of the plum pudding model? The primary limitation of the plum pudding model was its inability to account for the results of Rutherford’s gold foil experiment, which showed that the positive charge in an atom is concentrated in a small nucleus rather than being spread out. The model also did not accurately describe the arrangement of electrons around the nucleus.
9. How did the transition from the plum pudding model to the Rutherford model occur? The transition occurred as a result of Rutherford’s gold foil experiment, which demonstrated that the atom’s positive charge and most of its mass are concentrated in a central nucleus. This new understanding led to the development of the Rutherford model, which replaced the plum pudding model and laid the groundwork for future atomic theories.
10. Why is the plum pudding model still important today? The plum pudding model is important because it represents an early attempt to understand atomic structure and incorporates the concept of subatomic particles. It paved the way for subsequent atomic models and helped scientists develop a more accurate understanding of atomic structure.