Allotropy refers to the phenomenon where a chemical element exists in two or more different forms, called allotropes, in the same physical state. These different forms arise due to variations in the atomic arrangement or bonding of the element, which results in distinct physical and chemical properties for each allotrope.
Allotropy is an important concept in chemistry and materials science, as the same element can exhibit dramatically different behaviors depending on its allotrope.
Definition and Characteristics
- Allotropes: Allotropes are different structural forms of the same element, each with its unique physical or chemical properties. The differences arise from variations in the arrangement of atoms or the types of bonds between them.
- Physical State: Allotropy typically occurs in the solid state but can also occur in other states, such as gases (e.g., oxygen and ozone).
The changes in bonding and structure lead to different properties such as density, hardness, electrical conductivity, and color, even though the chemical element is the same.
Examples of Allotropes
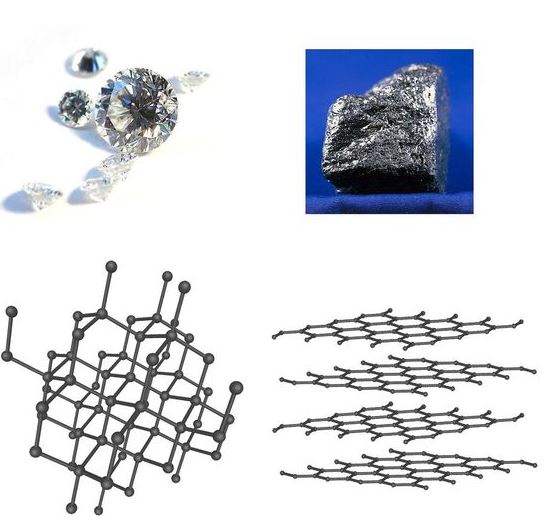
1. Carbon
Carbon is one of the most well-known elements with allotropes, with each form having significantly different physical properties:
- Diamond: In diamond, carbon atoms are arranged in a three-dimensional tetrahedral structure, where each carbon atom is bonded to four others in a very strong covalent bond. This makes diamond extremely hard, transparent, and an excellent electrical insulator.
- Graphite: In graphite, carbon atoms are arranged in layers of hexagonal lattices. Each carbon atom is bonded to three others, leaving one electron free to move between layers, which makes graphite a good conductor of electricity. Graphite is soft and slippery because the layers can slide over each other.
- Graphene: A single layer of carbon atoms arranged in a hexagonal lattice, graphene is a highly conductive, flexible, and incredibly strong material.
- Fullerenes (C60): Fullerenes, also known as buckyballs, consist of carbon atoms arranged in a spherical or ellipsoidal structure. They have unique properties and are used in nanotechnology and materials science.
- Carbon Nanotubes: These are cylindrical structures made from rolled-up sheets of graphene. They exhibit remarkable mechanical strength and electrical conductivity.
2. Oxygen
Oxygen has two main allotropes that are important in chemistry and environmental science:
- Dioxygen (O₂): The most common allotrope, O₂, consists of two oxygen atoms bonded together. It is essential for respiration in most life forms and is found in the Earth’s atmosphere.
- Ozone (O₃): Ozone consists of three oxygen atoms bonded together. It forms a protective layer in the Earth’s atmosphere, absorbing harmful ultraviolet radiation from the Sun, but at ground level, it is a pollutant and a component of smog.
3. Phosphorus
Phosphorus exists in several allotropes, each with different chemical reactivity and stability:
- White Phosphorus: Consisting of P₄ tetrahedra, white phosphorus is highly reactive and toxic. It glows in the dark due to its reaction with oxygen and is used in chemical weapons and flares.
- Red Phosphorus: A more stable allotrope, red phosphorus is used in safety matches and fireworks. It does not ignite as easily as white phosphorus.
- Black Phosphorus: The least reactive allotrope, black phosphorus has a layered structure similar to graphite and exhibits semiconductor properties, making it of interest in materials science and electronics.
4. Sulfur
Sulfur has multiple allotropes, with the most common being:
- Rhombic Sulfur: The most stable form at room temperature, consisting of S₈ molecules arranged in a crystal lattice.
- Monoclinic Sulfur: A form of sulfur that is stable at higher temperatures and converts to rhombic sulfur as it cools.
5. Tin
Tin (Sn) exhibits allotropic transformation depending on temperature:
- White Tin (β-tin): The metallic form of tin, stable at room temperature, is soft and malleable.
- Gray Tin (α-tin): At temperatures below 13.2°C, tin transforms into a brittle, non-metallic allotrope known as gray tin, which has a diamond-like structure. This process, known as tin pest, can cause the degradation of tin objects in cold environments.
Importance of Allotropy
Allotropy has significant implications in various scientific and industrial fields due to the different properties of each allotrope:
1. Material Science
Different allotropes of the same element can have drastically different applications due to their unique properties. For example:
- Diamond is used in cutting tools and jewelry due to its hardness, while graphite is used in pencils and as a lubricant.
- Graphene and carbon nanotubes are revolutionizing electronics and nanotechnology due to their exceptional conductivity and strength.
2. Chemistry and Environmental Science
- Ozone (O₃) plays a crucial role in protecting the Earth from ultraviolet radiation, but it also poses health risks at ground level.
- Allotropes of phosphorus serve different purposes: white phosphorus is used in military applications, while red phosphorus is used in everyday items like matches.
3. Phase Transitions
The study of allotropy helps scientists understand how elements behave under different conditions, such as pressure and temperature. For example, the transformation of tin at low temperatures has practical implications for materials used in cold environments.
Allotropy is a fascinating and important phenomenon in chemistry, where an element can exist in multiple forms with distinct properties. Whether it’s the hardness of diamond versus the softness of graphite, or the reactive nature of white phosphorus compared to the stability of red phosphorus, allotropes demonstrate the wide range of behaviors that elements can exhibit. The study and application of allotropes have profound impacts on industries ranging from electronics to materials science, shaping how we use and understand these elements in both everyday and cutting-edge technologies.