Solids are one of the fundamental states of matter, characterized by their fixed shape and volume. In material science, understanding solids is crucial because their structure determines their physical properties, which directly affect their practical uses. Solids can be broadly divided into two main categories based on the arrangement of their particles: crystalline and amorphous.
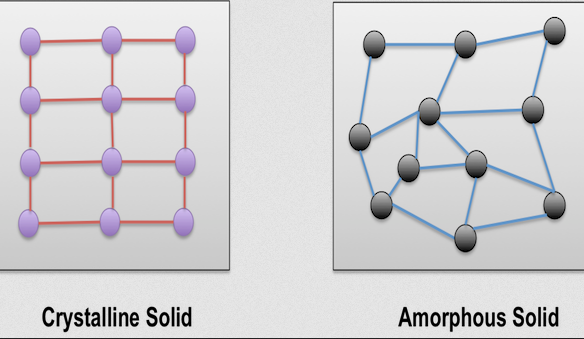
Crystalline solids have a highly ordered atomic structure, with particles arranged in a repeating, geometric pattern. In contrast, amorphous solids lack this long-range order and have a random arrangement of particles. The distinction between these two types of solids is critical for understanding their behavior in various applications, from electronics to construction materials. By examining their structure, scientists and engineers can manipulate materials to meet specific requirements, making it essential to differentiate between crystalline and amorphous solids.
1: What are Crystalline Solids?
Definition
Crystalline solids are materials in which the atoms, ions, or molecules are arranged in a highly ordered, repeating pattern that extends throughout the entire solid. This precise organization gives rise to distinct geometrical shapes and makes crystalline solids easily recognizable.
Key Characteristics
- Definite Geometric Shape
Crystalline solids often form well-defined shapes with flat surfaces and specific angles between them. This geometric precision is a result of the orderly arrangement of their constituent particles. - Sharp Melting Point
A defining feature of crystalline solids is their sharp and well-defined melting point. This means they transition from a solid to a liquid at a specific temperature, due to the uniform arrangement of particles. - Anisotropy
Crystalline solids are anisotropic, meaning their physical properties, such as thermal and electrical conductivity, vary depending on the direction in which they are measured within the crystal. This directional dependence is a result of the organized internal structure. - Long-range Order
The most significant feature of crystalline solids is their long-range order. Atoms or molecules in these solids are arranged in a consistent, repeating pattern that extends over large distances, giving them their stability and defining their physical properties.
2: What are Amorphous Solids?
Definition
Amorphous solids are materials in which the atoms, ions, or molecules are arranged randomly, lacking the regular, repeating structure seen in crystalline solids. Because of this disordered arrangement, amorphous solids do not exhibit long-range order, though they may have some short-range order where particles are slightly organized in local regions.
Key Characteristics
- No Definite Shape
Unlike crystalline solids, amorphous solids do not form well-defined geometric shapes. Their structure is irregular and lacks the flat surfaces and precise angles characteristic of crystals. - Gradual Melting Point
Amorphous solids do not have a sharp melting point. Instead, they soften gradually over a range of temperatures. This behavior occurs because their disordered atomic arrangement does not provide a consistent structure that can melt at a specific temperature. - Isotropy
Amorphous solids are isotropic, meaning their properties are the same in all directions. Since the atomic arrangement is random and lacks a defined structure, there is no directional dependence in physical properties like thermal or electrical conductivity. - Short-range Order
While amorphous solids lack long-range atomic order, they may exhibit some level of short-range order. This means that atoms or molecules are somewhat organized within small, localized regions, but this order does not extend throughout the entire material.
Examples of Amorphous Solids
Common examples include:
- Glass: A material typically made from silica (SiO₂), which lacks the crystalline structure seen in quartz.
- Rubber: A flexible material with a random arrangement of polymer chains.
- Plastics: Synthetic polymers with disordered structures that allow for flexibility and versatility.
Comparison to Supercooled Liquids
Amorphous solids are sometimes referred to as “supercooled liquids” because their atomic structure resembles that of liquids more than crystalline solids. However, unlike liquids, amorphous solids retain a solid form under normal conditions, even though their molecular structure is less rigid.
3: Crystalline vs. Amorphous Solids
| Property | Crystalline Solids | Amorphous Solids |
|---|---|---|
| Atomic Arrangement | Highly ordered, long-range order | Random and disordered, short-range order |
| Shape | Definite, geometric | No regular shape |
| Melting Point | Sharp, definite | Gradual softening over a range of temperatures |
| Anisotropy vs. Isotropy | Anisotropic (properties vary by direction) | Isotropic (properties are the same in all directions) |
| Examples | Metals, salts, diamonds | Glass, rubber, plastics |
4: Applications of Crystalline and Amorphous Solids
Practical Uses of Crystalline Solids
Crystalline solids play a crucial role in various industries and technologies due to their predictable and stable structure. Some notable applications include:
- Metals
Metals like iron, copper, and aluminum, which have crystalline structures, are widely used in construction, machinery, and electronics. Their ordered atomic arrangement contributes to their strength, ductility, and electrical conductivity, making them ideal for structural components and wiring. - Semiconductors
Crystalline solids such as silicon and germanium are essential in the electronics industry. Their well-defined atomic structure allows for precise control of electrical properties, which is critical in the manufacturing of microchips and transistors. - Crystals in Optics
Crystals like quartz and sapphire are used in optical devices such as lenses, lasers, and oscillators. Their orderly arrangement allows for the efficient transmission and manipulation of light. - Ionic Crystals
Substances like sodium chloride (table salt) and other ionic compounds are important in both industrial chemical processes and food preservation due to their stable crystal lattice.
Practical Uses of Amorphous Solids
Amorphous solids also have significant applications, particularly in products that require flexibility and variability in structure:
- Glass
One of the most common amorphous solids, glass is used in windows, containers, and optical fibers. Its transparency, durability, and ease of shaping make it invaluable in both everyday and specialized industrial applications. - Rubber
Amorphous rubber, with its random molecular structure, is essential in products requiring elasticity, such as tires, seals, and hoses. Its ability to stretch and return to shape is due to its disordered arrangement. - Plastics
Amorphous plastics like polyethylene and polystyrene are used in everything from packaging materials to household goods. Their versatility comes from their flexible, non-crystalline structure, allowing them to be molded into various shapes.
Conclusion
Crystalline and amorphous solids represent two distinct types of materials based on their atomic arrangement. Crystalline solids are characterized by their ordered structure, sharp melting point, and directional properties, while amorphous solids have a random atomic arrangement, gradual melting points, and isotropic properties.
Understanding the differences between these two forms is essential for their practical applications in science, technology, and everyday life. Crystalline materials are widely used in industries that require strength and precision, such as electronics and construction, while amorphous materials are valued for their flexibility and versatility, as seen in glass, rubber, and plastics. Both types of solids are integral to modern technology, and their distinct properties allow us to utilize them in ways that significantly impact our world.
FAQ
1. What is the main difference between crystalline and amorphous solids?
Crystalline solids have a highly ordered atomic structure with long-range order, resulting in well-defined shapes and sharp melting points. Amorphous solids, on the other hand, have a random, disordered atomic arrangement, lack a definite shape, and melt gradually over a range of temperatures.
2. Why do crystalline solids have sharp melting points?
Crystalline solids have a uniform, repeating atomic structure, so the entire solid transitions from solid to liquid at a specific temperature. This creates a distinct melting point.
3. Can a material change from crystalline to amorphous or vice versa?
Yes, some materials can change between crystalline and amorphous forms under certain conditions. For example, heating and rapidly cooling a crystalline solid can sometimes result in an amorphous form, such as in the case of glass from molten silica.
4. Why are crystalline solids anisotropic while amorphous solids are isotropic?
Crystalline solids are anisotropic because their ordered structure causes physical properties to vary depending on the direction of measurement. Amorphous solids are isotropic because their random atomic arrangement leads to uniform properties in all directions.
5. What are some common examples of crystalline solids?
Common examples of crystalline solids include metals like iron and copper, ionic compounds such as sodium chloride (table salt), and covalent crystals like diamonds.
6. What are some common examples of amorphous solids?
Examples of amorphous solids include glass, rubber, and plastics. These materials do not have a regular atomic structure and often exhibit flexibility or transparency.
7. Why are amorphous solids sometimes called “supercooled liquids”?
Amorphous solids are sometimes referred to as supercooled liquids because their molecular arrangement resembles that of liquids, although they behave like solids under normal conditions. Their atoms are not arranged in a strict lattice, similar to how particles move in a liquid.
8. What are the practical applications of crystalline solids?
Crystalline solids are used in industries that require strength and precision. Metals are used in construction and machinery, while semiconductors like silicon are essential in electronics. Crystals such as quartz are also used in optical devices.
9. What are the practical applications of amorphous solids?
Amorphous solids like glass are used in windows, optical fibers, and containers. Rubber is used in tires and elastic products, and plastics are used in a wide range of products, from packaging to household items.
10. Which type of solid, crystalline or amorphous, is better for making semiconductors?
Crystalline solids, particularly silicon, are better suited for making semiconductors because their orderly atomic structure allows for precise control of electrical properties, which is essential in electronic devices.
